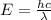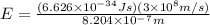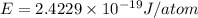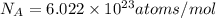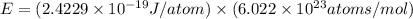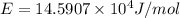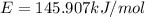Chemistry, 12.07.2019 19:30 yarrito20011307
Assume a hydrogen atom has an electron in the n-level corresponding to the month of your birthday (jan = 1, feb = 2, etc.) calculate the ionization energy of that hydrogen atom. hint: the ionization energy is defined as the energy needed to completely remove an electron from an atom, ion, or molecule.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Assume a hydrogen atom has an electron in the n-level corresponding to the month of your birthday (j...
Questions








Mathematics, 28.08.2019 13:30

History, 28.08.2019 13:30

Chemistry, 28.08.2019 13:30

Mathematics, 28.08.2019 13:30




History, 28.08.2019 13:30

History, 28.08.2019 13:30

Biology, 28.08.2019 13:30

Mathematics, 28.08.2019 13:30

Chemistry, 28.08.2019 13:30

History, 28.08.2019 13:30

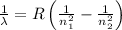
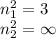
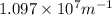
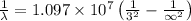
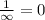
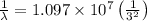
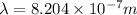
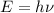 and
and 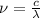
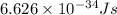 (Planck's constant)
(Planck's constant)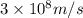 (Speed of light)
(Speed of light) in Energy formula, we get
in Energy formula, we get