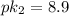Chemistry, 12.07.2019 18:00 maheshwarlall
He pka values of a compound with two ionizable groups are pk1 = 4.10 and pk2 between 7 and 10. a biochemist has 10 ml of a 1.0 m solution of this compound at a ph of 8.00. she adds 10.0 ml of 1.00 m hcl, which changes the ph to 3.20. what is pk2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
He pka values of a compound with two ionizable groups are pk1 = 4.10 and pk2 between 7 and 10. a bio...
Questions

Mathematics, 17.12.2021 07:30



English, 17.12.2021 07:30


Mathematics, 17.12.2021 07:30


Physics, 17.12.2021 07:30



Mathematics, 17.12.2021 07:30



Physics, 17.12.2021 07:30




History, 17.12.2021 07:30


Advanced Placement (AP), 17.12.2021 07:30

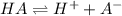
![pH = pk_a+ log \frac{[A^{-}]}{[HA]}](/tpl/images/0081/8142/50125.png)
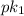 is:
is:![pH = pk_1+ log \frac{[A^{-}]}{[HA]}](/tpl/images/0081/8142/76eb5.png)
![3.2 = 4.1 + log \frac{[A^{-}]}{[HA]}](/tpl/images/0081/8142/89743.png)
![log \frac{[A^{-}]}{[HA]} = - 0.9](/tpl/images/0081/8142/41786.png)
![\frac{[A^{-}]}{[HA]} = 0.126](/tpl/images/0081/8142/5103f.png)
![pH = pk_2+ log \frac{[A^{-}]}{[HA]}](/tpl/images/0081/8142/126cd.png)