
Chemistry, 12.07.2019 18:00 darkghostmist
The unknowns are a mixture of ferrous ammonium sulfate and ammonium sulfate. calculate the amount of ferrous ammonium sulfate and the amount of ammonium sulfate needed to prepare a sample that is 10% fe with a total mass of 0.2 g

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2
You know the right answer?
The unknowns are a mixture of ferrous ammonium sulfate and ammonium sulfate. calculate the amount of...
Questions

Physics, 25.06.2019 19:00



History, 25.06.2019 19:00


History, 25.06.2019 19:00

Mathematics, 25.06.2019 19:00


History, 25.06.2019 19:00

Mathematics, 25.06.2019 19:00


Mathematics, 25.06.2019 19:00

Mathematics, 25.06.2019 19:00




Biology, 25.06.2019 19:00

Social Studies, 25.06.2019 19:00


Social Studies, 25.06.2019 19:00

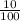 x0.2
x0.2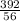 x 0.02
x 0.02

