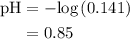Chemistry, 12.07.2019 11:30 brisamauro27
A50.0-ml sample of 0.50 m hcl is titrated with 0.50 m naoh. what is the ph of the solution after 28.0 ml of naoh have been added to the acid? 14) a.2.85 b.0.85 c.2.96 d.3.81 e.1.49

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

You know the right answer?
A50.0-ml sample of 0.50 m hcl is titrated with 0.50 m naoh. what is the ph of the solution after 28....
Questions



Mathematics, 29.03.2021 23:50

History, 29.03.2021 23:50

Chemistry, 29.03.2021 23:50

Computers and Technology, 29.03.2021 23:50





Mathematics, 29.03.2021 23:50


Mathematics, 29.03.2021 23:50





Computers and Technology, 29.03.2021 23:50

Mathematics, 29.03.2021 23:50

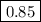 .
.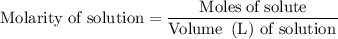
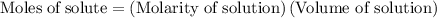 ......(2)
......(2)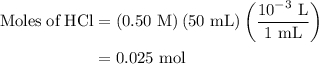
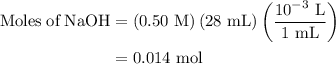
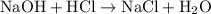
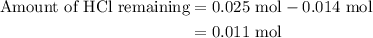
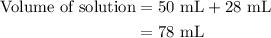
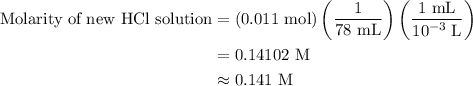
![{\text{pH}}=- {\text{log}}\left[ {{{\text{H}}^ + }}\right]](/tpl/images/0080/7989/2a671.png) ......(3)
......(3)![\left[{{{\text{H}}^ + }}\right]](/tpl/images/0080/7989/0bedd.png) is hydrogen ion concentration.
is hydrogen ion concentration.