

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1
You know the right answer?
Write the balanced chemical equations for the decomposition of solid calcium hydroxide into solid ca...
Questions


Mathematics, 03.02.2020 02:53






Mathematics, 03.02.2020 02:53

Mathematics, 03.02.2020 02:53



Mathematics, 03.02.2020 02:53


Chemistry, 03.02.2020 02:53

Chemistry, 03.02.2020 02:53


Biology, 03.02.2020 02:53

Mathematics, 03.02.2020 02:53

Mathematics, 03.02.2020 02:53

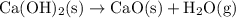 .
.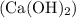 decomposes to form solid calcium (ii) oxide (lime)
decomposes to form solid calcium (ii) oxide (lime) 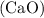 and water vapour
and water vapour 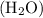
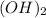 in to lime (CaO) and water (
in to lime (CaO) and water (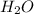 at high temperature. The reaction is an endothermic reaction. That is heat is absorbed in this reaction process. One mole of calcium hydroxide decomposed into one mole of calcium oxide and one mole of water. The balanced reaction can be shown as-CaC
at high temperature. The reaction is an endothermic reaction. That is heat is absorbed in this reaction process. One mole of calcium hydroxide decomposed into one mole of calcium oxide and one mole of water. The balanced reaction can be shown as-CaC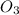 (solid) → CaO (solid) +
(solid) → CaO (solid) + 

