
If the same quantity of heat is added to both a 2-liter and a 4-liter container of water, the temperature change for water in the 4-liter container will be a. half that of the 2-liter container. b. more than half but less than twice. c. twice that of the 2-liter container. d. no change. explain.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 12:30
What would happen to a weak base dissociation equilibrium if more products we added
Answers: 1

Chemistry, 23.06.2019 12:50
Complete the paragraph to describe the characteristics of a borane molecule (bh3). the lewis structure and table of electronegativities are given. the bond polarities in bh3 are , the molecular shape is , and the molecule is .
Answers: 2

Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1
You know the right answer?
If the same quantity of heat is added to both a 2-liter and a 4-liter container of water, the temper...
Questions





Arts, 19.01.2021 22:20


Mathematics, 19.01.2021 22:20


Biology, 19.01.2021 22:20

Mathematics, 19.01.2021 22:20


Mathematics, 19.01.2021 22:20



French, 19.01.2021 22:20

English, 19.01.2021 22:20


English, 19.01.2021 22:20

Medicine, 19.01.2021 22:20

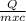
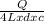
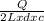
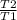 = 2
= 2

