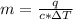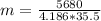Chemistry, 10.07.2019 07:30 katiems5514
Akettle of water is at 14.5°c. its temperature is then raised to 50.0°c by supplying it with 5,680 joules of heat. the specific heat capacity of water is 4.186 joules/gram degree celsius. what is the mass of water in the kettle? express your answer to three significant figures. the mass of the water in the kettle is grams.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1
You know the right answer?
Akettle of water is at 14.5°c. its temperature is then raised to 50.0°c by supplying it with 5,680 j...
Questions


Mathematics, 12.02.2020 20:59


Mathematics, 12.02.2020 20:59



Business, 12.02.2020 20:59


Mathematics, 12.02.2020 20:59



History, 12.02.2020 20:59

English, 12.02.2020 20:59

History, 12.02.2020 20:59

Social Studies, 12.02.2020 20:59

English, 12.02.2020 20:59




Mathematics, 12.02.2020 20:59

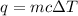
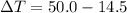 = 35.5 degree C
= 35.5 degree C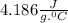 .
.