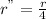Chemistry, 10.07.2019 05:30 jessie8022
Is first order in bro3⎻ , second order in br⎻, and zero order in h+. by what factor will the reaction rate change if the concentration of bro3⎻ is doubled, the concentration of br⎻ is halved, and the concentration of h+ is tripled ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3


Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Is first order in bro3⎻ , second order in br⎻, and zero order in h+. by what factor will the reactio...
Questions

Chemistry, 25.07.2019 11:00


Chemistry, 25.07.2019 11:00



History, 25.07.2019 11:00

History, 25.07.2019 11:00

Social Studies, 25.07.2019 11:00

History, 25.07.2019 11:00

History, 25.07.2019 11:00


Mathematics, 25.07.2019 11:00


Chemistry, 25.07.2019 11:00


History, 25.07.2019 11:00


Business, 25.07.2019 11:00


Chemistry, 25.07.2019 11:00

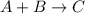
![r=k[A]^{a}[B]^{b}](/tpl/images/0072/1558/66bfa.png)
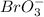 , second order with respect to
, second order with respect to 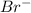 and zero order with respect to
and zero order with respect to 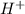 .
.![r=k[BrO_{3}^{-}]^{1}[Br^{-}]^{2}[H^{+}]^{0}](/tpl/images/0072/1558/0a846.png)
![r=k[BrO_{3}^{-}][Br^{-}]^{2}](/tpl/images/0072/1558/30a30.png) ...... (1)
...... (1)![r^{'}=2k[BrO_{3}^{-}][Br^{-}]^{2}](/tpl/images/0072/1558/4b879.png) ...... (2)
...... (2)![\frac{r^{'}}{r}=\frac{2k[BrO_{3}^{-}][Br^{-}]^{2}}{k[BrO_{3}^{-}][Br^{-}]^{2}}=2](/tpl/images/0072/1558/5cd5e.png)
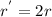
^{2}](/tpl/images/0072/1558/6cb53.png)
![r^{"}=1/4k[BrO_{3}^{-}][Br^{-}]^{2}](/tpl/images/0072/1558/92754.png) ...... (3)
...... (3)![\frac{r^{"}}{r}=\frac{1/4k[BrO_{3}^{-}][Br^{-}]^{2}}{k[BrO_{3}^{-}][Br^{-}]^{2}}=\frac{1}{4}](/tpl/images/0072/1558/807de.png)