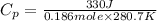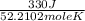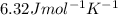Chemistry, 09.07.2019 21:00 maxi12312345
It takes 330 j of energy to raise the temperature of 14.6 g of benzene from 21.0 °c to 28.7 °c at constant pressure. what is the constant-pressure molar heat capacity of benzene?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
It takes 330 j of energy to raise the temperature of 14.6 g of benzene from 21.0 °c to 28.7 °c at co...
Questions

Arts, 26.11.2020 08:20

Physics, 26.11.2020 08:20

Social Studies, 26.11.2020 08:20


French, 26.11.2020 08:20

Health, 26.11.2020 08:20

Mathematics, 26.11.2020 08:20



Social Studies, 26.11.2020 08:20




History, 26.11.2020 08:20

Mathematics, 26.11.2020 08:20



Computers and Technology, 26.11.2020 08:20

Mathematics, 26.11.2020 08:20

Mathematics, 26.11.2020 08:20

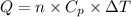 (1)
(1)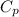 = molar heat capacity at constant pressure
= molar heat capacity at constant pressure = change in temperature
= change in temperature 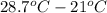
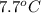
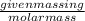
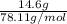
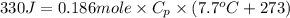 (conversion of degree Celsius into kelvin)
(conversion of degree Celsius into kelvin)