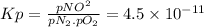Chemistry, 06.07.2019 06:30 NateTheBeast12
The equilibrium constant (kp) for the formation of the air pollutant nitric oxide (no) in an automobile engine at 537°c is 4.5 × 10−11. n2(g) + o2(g) ⇌ 2no(g) (a) calculate the partial pressure of no under these conditions if the partial pressures of nitrogen and oxygen are 3.00 and 0.012 atm, respectively.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
The equilibrium constant (kp) for the formation of the air pollutant nitric oxide (no) in an automob...
Questions

Mathematics, 23.10.2020 02:01


Chemistry, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01



English, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01


History, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01

Chemistry, 23.10.2020 02:01

Biology, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01

English, 23.10.2020 02:01


Mathematics, 23.10.2020 02:01

Chemistry, 23.10.2020 02:01

Mathematics, 23.10.2020 02:01

Health, 23.10.2020 02:01