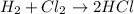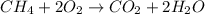Chemistry, 05.07.2019 12:30 izzysmith6836
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2 + 2h2o equation a represents a reaction, and equation b represents reaction. choices for both blanks combustion decombustion synthesis double displacement single displacement choose one for each blank there are some notes and examples in the images my opinions are 1. 2blank--combustion tell me if im correct 16pionts

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
You know the right answer?
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2...
Questions


Social Studies, 05.08.2019 22:20


Mathematics, 05.08.2019 22:20


Mathematics, 05.08.2019 22:20

Mathematics, 05.08.2019 22:20


Mathematics, 05.08.2019 22:20

Law, 05.08.2019 22:20







Mathematics, 05.08.2019 22:20