
Chemistry, 04.07.2019 21:30 lexipeacemaker
Noble gases (18): (name all noble gases) • these elements do not easily combine with other elements. they are odorless, colorless and therefore were difficult to discover. • when air is cold, oxygen liquefies first at -18o celsius. about 1% of the air is still gas composed of the noble gas entire noble gas short version of noble gas • one noble gas was discovered on the sun. it’s spectrum was identify first. this is • one of noble gas is radioactive and seeps up your basement floors and walls. it is • this noble gas is used in electronic flashes and other bright lights. it is• this noble gas is best known for its orange color in gas discharge tubes. it is used for signs that bear its name. it is

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 23.06.2019 10:30
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1

Chemistry, 23.06.2019 11:00
Which of the following reactions is endothermic? h2(g) + ½ o2(g) h2o(g), h = -57.82 kcal ½n2(g) + o2(g) + 8.1 kcal no2(g) ½ n2(g) + 3/2 h2(g) nh3(g) + 11.0 kcal c(diamond) + o2(g) co2, h = -94.50 kcal
Answers: 2
You know the right answer?
Noble gases (18): (name all noble gases) • these elements do not easily combine with other element...
Questions


English, 05.09.2020 01:01




Social Studies, 05.09.2020 01:01


Business, 05.09.2020 01:01



English, 05.09.2020 01:01



Mathematics, 05.09.2020 01:01

Mathematics, 05.09.2020 01:01

Social Studies, 05.09.2020 01:01

English, 05.09.2020 01:01



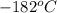 .
. 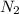 liquefies at
liquefies at 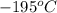 . About 1% of the air is still gas composed of the noble gas Argon, Ar.One noble gas was discovered on the sun. It’s spectrum was identify first. This is Helium, He.One of noble gas is radioactive and seeps up your basement floors and walls. It is Radon, Rn.This noble gas is used in electronic flashes and other bright lights. It is Xenon, Xe.This noble gas is best known for its orange color in gas discharge tubes. It is used for signs that bear its name. It is Neon, Ne.
. About 1% of the air is still gas composed of the noble gas Argon, Ar.One noble gas was discovered on the sun. It’s spectrum was identify first. This is Helium, He.One of noble gas is radioactive and seeps up your basement floors and walls. It is Radon, Rn.This noble gas is used in electronic flashes and other bright lights. It is Xenon, Xe.This noble gas is best known for its orange color in gas discharge tubes. It is used for signs that bear its name. It is Neon, Ne.

