
Chemistry, 04.07.2019 10:00 kenken2583
A50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorimeter filled with water. the calorimeter and the water have a combined mass of 250.0 g and an overall specific heat of 1.035 cal/g•°c. the initial temperature of the calorimeter is 10.00°c. the system reaches a final temperature of 11.08°c when the metal is added. any heat lost by the metal is

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1


Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

You know the right answer?
A50.00 g sample of an unknown metal is heated to 45.00°c. it is then placed in a coffee-cup calorime...
Questions


Mathematics, 21.04.2020 17:35



Mathematics, 21.04.2020 17:35


Computers and Technology, 21.04.2020 17:35



Mathematics, 21.04.2020 17:35




Mathematics, 21.04.2020 17:35

Mathematics, 21.04.2020 17:35

SAT, 21.04.2020 17:35

Mathematics, 21.04.2020 17:35

English, 21.04.2020 17:35

History, 21.04.2020 17:35

Mathematics, 21.04.2020 17:35

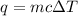 .
. is change in temperature.
is change in temperature.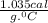 .
. 

