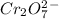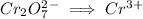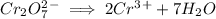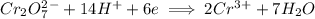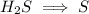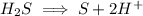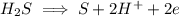Chemistry, 03.07.2019 01:00 babyboo6745
Write the half reactions for the following equation. identify which half reaction is oxidized and which is reduced. ag + no₃- --> ag+ + no write the half reactions for the following equation. identify which half reaction is oxidized and which is reduced. zn + no₃- --> zn²+ + no₂ write the half reactions for the following equation. identify which half reaction is oxidized and which is reduced. cr₂o₇ ²- (aq) + h₂s(g) --> cr³+(aq) + s(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2



You know the right answer?
Write the half reactions for the following equation. identify which half reaction is oxidized and w...
Questions





History, 05.11.2019 23:31



History, 05.11.2019 23:31




Social Studies, 05.11.2019 23:31

Social Studies, 05.11.2019 23:31

Mathematics, 05.11.2019 23:31




English, 05.11.2019 23:31


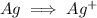
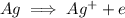
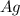 is being oxidized. Oxidation is loss of electrons.
is being oxidized. Oxidation is loss of electrons. 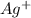
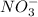

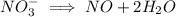
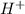 ions on left hand side to balance hyrdogen.
ions on left hand side to balance hyrdogen.
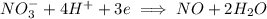
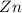
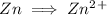
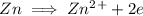
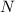 is being reduced since it is moving from a +5 oxidation state to a +2 oxidation state by gaining electrons.
is being reduced since it is moving from a +5 oxidation state to a +2 oxidation state by gaining electrons.