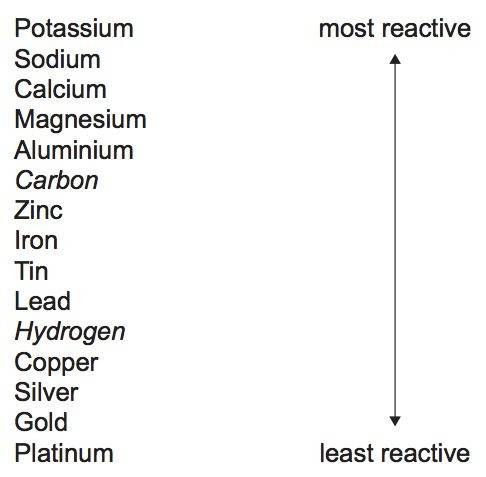
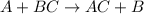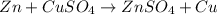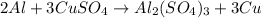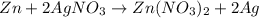30 pts! balancing equations. 2. for part 2: single-displacement reactions: for each of the four single-displacement reactions, describe what happened in each well. if a chemical reaction occurred, write a balanced equation for it. then using the a, b symbols, write a general equation for a single-displacement reaction. a+bc=ac+b here are the chemical formulas of the reactants for each reaction: • zinc – zn copper sulfate – cuso4 zn+cuso4-> cu+znso4 • aluminum – al copper sulfate – cuso4 no reaction • zinc – zn silver nitrate – ag(no3) • copper – cu silver nitrate – ag(no3)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1



Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
30 pts! balancing equations. 2. for part 2: single-displacement reactions: for each of the four s...
Questions

Mathematics, 30.08.2020 14:01



English, 30.08.2020 14:01

Biology, 30.08.2020 14:01

Social Studies, 30.08.2020 14:01

Mathematics, 30.08.2020 14:01

Chemistry, 30.08.2020 14:01

Social Studies, 30.08.2020 14:01

Chemistry, 30.08.2020 14:01

English, 30.08.2020 14:01

Mathematics, 30.08.2020 14:01

Mathematics, 30.08.2020 14:01

English, 30.08.2020 14:01

English, 30.08.2020 14:01

History, 30.08.2020 14:01

Biology, 30.08.2020 14:01

Physics, 30.08.2020 14:01

English, 30.08.2020 14:01

Mathematics, 30.08.2020 14:01