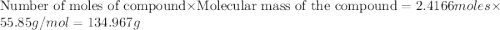Chemistry, 01.07.2019 08:30 edeliz2804
Al (s) + fe2o3 (s) --> al2o3 (s) + fe (s) (needs balancing) how many grams of fe can be produced when 65.2 g of al is reacted with an excess (unlimited) supply of fe2o3?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2


Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
Al (s) + fe2o3 (s) --> al2o3 (s) + fe (s) (needs balancing) how many grams of fe can be produced...
Questions





Mathematics, 17.04.2020 23:11










Computers and Technology, 17.04.2020 23:14


Mathematics, 17.04.2020 23:14


Computers and Technology, 17.04.2020 23:14

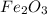 .
.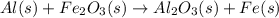
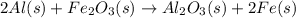
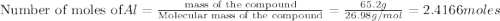
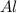 reacts with one mole of
reacts with one mole of 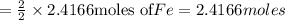
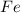 produced =
produced =