

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

You know the right answer?
What is the numerical value of kc for the following reaction if the equilibrium mixture contains 0.5...
Questions

History, 19.07.2019 17:00


Social Studies, 19.07.2019 17:00

Physics, 19.07.2019 17:00

Biology, 19.07.2019 17:00

Physics, 19.07.2019 17:00


Mathematics, 19.07.2019 17:00

Physics, 19.07.2019 17:00









Biology, 19.07.2019 17:00

Spanish, 19.07.2019 17:00

Social Studies, 19.07.2019 17:00

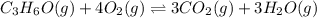
![Kc=\frac{[CO_2]^3[H_2O]^3}{[C_3H_6O][O_2]^4}](/tpl/images/0036/7459/52f34.png)
![Kc=\frac{[1.8]^3[2.0]^3}{[0.51][0.30]^4}](/tpl/images/0036/7459/c5aa6.png)


