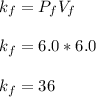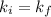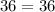Acertain gas is present in a 12.0l cylinder at 3.0 atm pressure. if the pressure is increased to 6.0 atm, the volume of the gas decreases to 6.0l. find the two constants ki, the initial value of k, and kf, the final value of k, to verify if whether the gas obeys boyle’s law.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
Acertain gas is present in a 12.0l cylinder at 3.0 atm pressure. if the pressure is increased to 6.0...
Questions

History, 21.02.2021 14:00


English, 21.02.2021 14:00

Chemistry, 21.02.2021 14:00

Geography, 21.02.2021 14:00

Chemistry, 21.02.2021 14:00

Chemistry, 21.02.2021 14:00


History, 21.02.2021 14:00


Mathematics, 21.02.2021 14:00

History, 21.02.2021 14:00

Mathematics, 21.02.2021 14:00


Mathematics, 21.02.2021 14:00

English, 21.02.2021 14:00

English, 21.02.2021 14:00

Chemistry, 21.02.2021 14:00


English, 21.02.2021 14:00

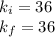
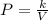
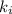 :
: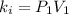
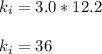
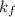 :
: