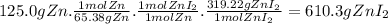Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1

Chemistry, 23.06.2019 13:00
Write the balanced chemical reaction for the formation of fe2(so4)3 from fe2o3 and so3 and determine how many moles of fe2(so4)3 are formed when 12.7 mol of so3 are reacted.
Answers: 1

Chemistry, 23.06.2019 13:00
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2
You know the right answer?
Zinc reacts with iodine in a synthesis reaction. using a balanced chemical equation for the reaction...
Questions


Biology, 12.11.2020 23:10


History, 12.11.2020 23:10

Health, 12.11.2020 23:10








Mathematics, 12.11.2020 23:10




History, 12.11.2020 23:10



Health, 12.11.2020 23:10