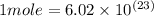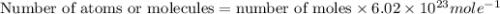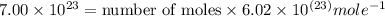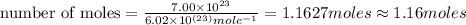Chemistry, 28.06.2019 20:30 truthqmatic16
In the world of chemistry, a 'mole' is a number of a very large number of something. just as a 'dozen' is 12 of something, a 'mole' is about 6.02 * 10^23 of something. in chemistry, we like to measure the number of atoms or molecules in moles. if one mole is equal to 6.02 * 10^23 atoms, and you have 7.00 * 10^23 atoms, then how many moles do you have? round your answer to two decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
In the world of chemistry, a 'mole' is a number of a very large number of something. just as a 'do...
Questions



Mathematics, 01.09.2020 06:01

Computers and Technology, 01.09.2020 06:01

Mathematics, 01.09.2020 06:01

Physics, 01.09.2020 06:01

Mathematics, 01.09.2020 06:01

Mathematics, 01.09.2020 06:01

Mathematics, 01.09.2020 06:01

English, 01.09.2020 06:01

Biology, 01.09.2020 06:01

History, 01.09.2020 06:01

Spanish, 01.09.2020 06:01

Mathematics, 01.09.2020 06:01

Mathematics, 01.09.2020 06:01

Biology, 01.09.2020 06:01




Mathematics, 01.09.2020 06:01

 numbers of atoms.
numbers of atoms.