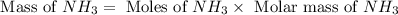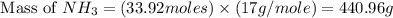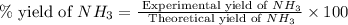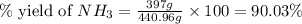Chemistry, 27.06.2019 20:30 elijahlylejamez45
25 i got the #1, just not #2 and #3. an industrial chemical company has opened a new plant that will produce ammonia (nh3). hydrogen and nitrogen gases are reacted to produce the ammonia. for the first batch of ammonia production, 475 g of nitrogen is reacted with excess hydrogen, and 397 g of ammonia are produced. • write the balanced equation for the formation of ammonia from hydrogen and nitrogen.  2nh3 • calculate the theoretical yield of ammonia. work must be shown to earn credit. • calculate the percent yield for the ammonia production. work must be shown to earn credit.
2nh3 • calculate the theoretical yield of ammonia. work must be shown to earn credit. • calculate the percent yield for the ammonia production. work must be shown to earn credit.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
25 i got the #1, just not #2 and #3. an industrial chemical company has opened a new plant that wil...
Questions


English, 24.09.2019 17:40


Mathematics, 24.09.2019 17:40


History, 24.09.2019 17:40



Mathematics, 24.09.2019 17:40


Physics, 24.09.2019 17:40

History, 24.09.2019 17:40

Physics, 24.09.2019 17:40

Mathematics, 24.09.2019 17:40

Mathematics, 24.09.2019 17:40

Computers and Technology, 24.09.2019 17:40

History, 24.09.2019 17:40

English, 24.09.2019 17:40

Mathematics, 24.09.2019 17:40

Social Studies, 24.09.2019 17:40

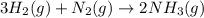
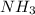 gas = 440.96 g
gas = 440.96 g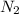 = 475 g
= 475 g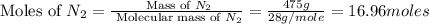
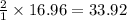 moles of
moles of