
Chemistry, 27.06.2019 19:30 ccarwile01
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. (4 points) a + b yieldsproducts trial [a] [b] rate 1 0.10 m 0.20 m 1.2 × 10-2 m/min 2 0.10 m 0.40 m 4.8 × 10-2 m/min 3 0.20 m 0.40 m 9.6 × 10-2 m/min

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2


Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
Determine the rate law, including the values of the orders and rate law constant, for the following...
Questions







Arts, 23.08.2021 23:20

Mathematics, 23.08.2021 23:20


English, 23.08.2021 23:20

Business, 23.08.2021 23:20

Mathematics, 23.08.2021 23:20

Social Studies, 23.08.2021 23:20


Health, 23.08.2021 23:20


Geography, 23.08.2021 23:20


Mathematics, 23.08.2021 23:20

Geography, 23.08.2021 23:20

![k[A]^1[B]^2](/tpl/images/0024/4376/f6c70.png) , order with respect to A is 1, order with respect to B is 2 and total order is 3. Rate law constant is
, order with respect to A is 1, order with respect to B is 2 and total order is 3. Rate law constant is 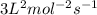
![Rate=k[A]^x[B]^y](/tpl/images/0024/4376/ddde1.png)
![1.2\times 10^{-2}=k[0.10]^x[0.20]^y](/tpl/images/0024/4376/c938a.png) (1)
(1)![4.8\times 10^{-2}=k[0.10]^x[0.40]^y](/tpl/images/0024/4376/a7ba9.png) (2)
(2)![\frac{4.8\times 10^{-2}}{1.2\times 10^{-2}}=\frac{k[0.10]^x[0.40]^y}{k[0.10]^x[0.20]^y}](/tpl/images/0024/4376/b4c1f.png)
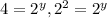 therefore y=2.
therefore y=2.![9.6\times 10^{-2}=k[0.20]^x[0.40]^y](/tpl/images/0024/4376/18d47.png) (4)
(4)![\frac{9.6\times 10^{-2}}{4.8\times 10^{-2}}=\frac{k[0.20]^x[0.40]^y}{k[0.10]^x[0.40]^y}](/tpl/images/0024/4376/61313.png)
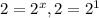 , x=1
, x=1![Rate=k[A]^1[B]^2](/tpl/images/0024/4376/ca297.png)
![1.2\times 10^{-2}=k[0.10]^1[0.20]^2](/tpl/images/0024/4376/f6a0d.png)
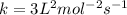 .
.

