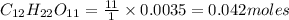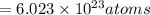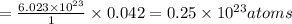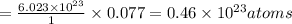Chemistry, 27.06.2019 07:00 probablyacommunist
2. a sample of table sugar (sucrose, c12h22o11) has a mass of 1.202 g. a. calculate the number of moles of c12h22o11 contained in the sample and record in table 1. show your work. b. calculate the moles of each element in c12h22o11 and record in table 1. show your work. c. calculate the number of atoms of each type in c12h22o11 and record in table 1. show your work. show your work and calulation.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
2. a sample of table sugar (sucrose, c12h22o11) has a mass of 1.202 g. a. calculate the number of mo...
Questions

History, 16.03.2020 23:51

English, 16.03.2020 23:51



Chemistry, 16.03.2020 23:51




Mathematics, 16.03.2020 23:51

Advanced Placement (AP), 16.03.2020 23:51


Mathematics, 16.03.2020 23:51






Mathematics, 16.03.2020 23:51


Mathematics, 16.03.2020 23:51

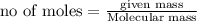
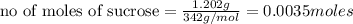
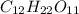 = 12 moles
= 12 moles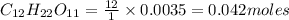
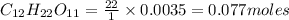
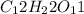 = 11 moles
= 11 moles