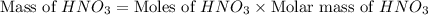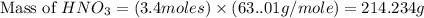Chemistry, 26.06.2019 01:30 nculberson6
Calculate the mass of 3.4 moles of nitric acid (hno3). explain the process or show your work by including all values used to determine the answer

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
Calculate the mass of 3.4 moles of nitric acid (hno3). explain the process or show your work by incl...
Questions

Mathematics, 09.07.2019 19:10


English, 09.07.2019 19:10

Biology, 09.07.2019 19:10

Physics, 09.07.2019 19:10


English, 09.07.2019 19:10


Physics, 09.07.2019 19:10




History, 09.07.2019 19:10


Chemistry, 09.07.2019 19:10


English, 09.07.2019 19:10



Mathematics, 09.07.2019 19:20