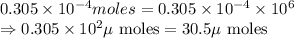Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
Achemist adds 50.0 ml of a 6.1 x 10^-4 m copper(ii) fluoride solution to a reaction flask. calculate...
Questions





Chemistry, 18.01.2022 08:40




Computers and Technology, 18.01.2022 08:40

Biology, 18.01.2022 08:40


Mathematics, 18.01.2022 08:40






Mathematics, 18.01.2022 08:50

Mathematics, 18.01.2022 08:50

Mathematics, 18.01.2022 08:50

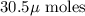
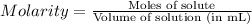
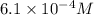
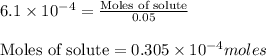
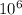
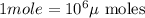
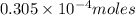 into micro-moles:
into micro-moles: