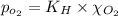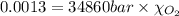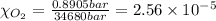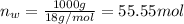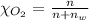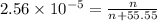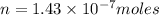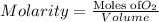Chemistry, 03.10.2019 05:50 yyyyyyyyy8938
Using henry's law, calculate the molar concentration of o2 in the surface water of a mountain lake saturated with air at 20 ∘c and an atmospheric pressure of 685 torr .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Using henry's law, calculate the molar concentration of o2 in the surface water of a mountain lake s...
Questions

Mathematics, 12.02.2020 04:39




Mathematics, 12.02.2020 04:39









Social Studies, 12.02.2020 04:39



Computers and Technology, 12.02.2020 04:39


Chemistry, 12.02.2020 04:39

Mathematics, 12.02.2020 04:39

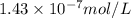 .
.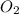 gas = 685 torr = 0.8905 bar
gas = 685 torr = 0.8905 bar