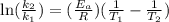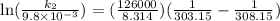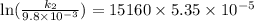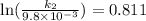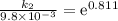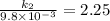Chemistry, 22.01.2020 23:31 19thomasar
The aquation of tris(1,10-phenanthroline)iron(ii) in acid solution takes place according to the equation: fe(phen)32+ + 3 h3o+ + 3 h2o → fe(h2o)62+ + 3 phenh+. if the activation energy, ea, is 126 kj/mol and the rate constant at 30°c is 9.8 × 10-3 min-1, what is the rate constant at 35°c? the aquation of tris(1,10-phenanthroline)iron(ii) in acid solution takes place according to the equation: fe(phen)32+ + 3 h3o+ + 3 h2o → fe(h2o)62+ + 3 phenh+. if the activation energy, ea, is 126 kj/mol and the rate constant at 30°c is 9.8 × 10-3 min-1, what is the rate constant at 35°c? 4.4 × 10-3 min-1 4.5 × 101 min-1 2.3 × 102 min-1 2.2 × 10-2 min-1

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
The aquation of tris(1,10-phenanthroline)iron(ii) in acid solution takes place according to the equa...
Questions




















Social Studies, 26.11.2019 01:31