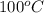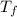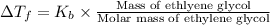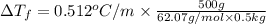Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1


Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1
You know the right answer?
Calculate the boiling point of a solution of 500.0 g of ethylene glycol (c2h6o2) dissolved in 500.0...
Questions

Computers and Technology, 29.09.2019 07:10

Mathematics, 29.09.2019 07:10



Physics, 29.09.2019 07:10


History, 29.09.2019 07:10

Mathematics, 29.09.2019 07:10

Mathematics, 29.09.2019 07:10

English, 29.09.2019 07:10


Mathematics, 29.09.2019 07:10

Mathematics, 29.09.2019 07:10




Arts, 29.09.2019 07:10

History, 29.09.2019 07:10


Spanish, 29.09.2019 07:10