Which of the following is true for the equilibrium constant of a reaction?
a. it is a ratio o...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3


Chemistry, 23.06.2019 08:50
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2
You know the right answer?
Questions


History, 07.01.2020 21:31







Geography, 07.01.2020 21:31



Mathematics, 07.01.2020 21:31


Health, 07.01.2020 21:31




Mathematics, 07.01.2020 21:31


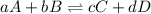
![K=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0433/4366/91a0a.png)


