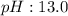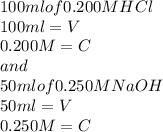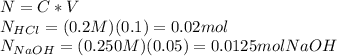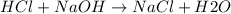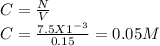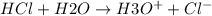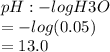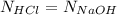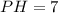Chemistry, 14.10.2019 00:30 angelisevega
100. ml of 0.200m hcl is titrated with 0.250m naoh.
1. what is the ph of the solution after 50.0ml of base has been added?
2.what is the ph of the solution at the equivalence point?

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
100. ml of 0.200m hcl is titrated with 0.250m naoh.
1. what is the ph of the solution after 50...
1. what is the ph of the solution after 50...
Questions



Mathematics, 15.10.2019 22:00


History, 15.10.2019 22:00




Social Studies, 15.10.2019 22:00



History, 15.10.2019 22:00

English, 15.10.2019 22:00

Mathematics, 15.10.2019 22:00



Spanish, 15.10.2019 22:00


Mathematics, 15.10.2019 22:00