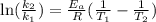Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

You know the right answer?
The activation energy of a certain reaction is 40.1 kj/mol . at 26 ∘c , the rate constant is 0.0160s...
Questions


English, 30.03.2021 06:30


Mathematics, 30.03.2021 06:30

Mathematics, 30.03.2021 06:30




History, 30.03.2021 06:30





Mathematics, 30.03.2021 06:30



Mathematics, 30.03.2021 06:30

Mathematics, 30.03.2021 06:30


Mathematics, 30.03.2021 06:30