Chemistry, 31.01.2020 13:56 sierravick123owr441
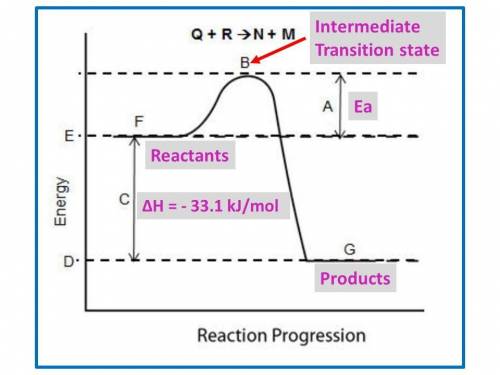
B. for the following questions, use the reaction no2(g) n2(g) + o2(g), with δh = –33.1 kj/mol and δs= 63.02 j/(mol·k).
i. draw a possible potential energy diagram of the reaction. label the enthalpy of the reaction.
ii. is the reaction endothermic or exothermic? explain your answer. (2 points)
iii. what is the gibbs free energy of the reaction at 25°c?
iv. is the reaction spontaneous or nonspontaneous at 25°c? explain your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1


Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
B. for the following questions, use the reaction no2(g) n2(g) + o2(g), with δh = –33.1 kj/mol and δs...
Questions


















Mathematics, 15.10.2019 02:20


History, 15.10.2019 02:20