Which formula can be used to calculate the molar mass of ammonia (nh3)?
a. molar mass o...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
Questions


Mathematics, 30.10.2019 04:31

History, 30.10.2019 04:31

Mathematics, 30.10.2019 04:31




Mathematics, 30.10.2019 04:31

Mathematics, 30.10.2019 04:31

Mathematics, 30.10.2019 04:31

Geography, 30.10.2019 04:31


Mathematics, 30.10.2019 04:31

Geography, 30.10.2019 04:31


Biology, 30.10.2019 04:31

Social Studies, 30.10.2019 04:31



History, 30.10.2019 04:31

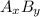
![[(x\times \text{Molar mass of A})+(y\times \text{Molar mass of B})]](/tpl/images/0480/1262/53b01.png)
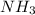 ,
,![[(1\times \text{Molar mass of Niyrogen})+(3\times \text{Molar mass of Hydrogen})]](/tpl/images/0480/1262/2b7d3.png)


