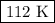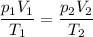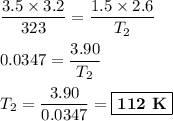Urgent 25 !
consider this gas law problem: if i have 3.2 l of gas at a pressure of 3.5 atm a...

Urgent 25 !
consider this gas law problem: if i have 3.2 l of gas at a pressure of 3.5 atm and a temperature of 323 k, what will be the temperature of the gas if i decrease the volume of the gas to 2.6 l and decrease the pressure to 1.5
answer all parts below for full credit:
a) what are the knows in this problem
b) what is the problem asking you to find
c) which gas law is the best law for finding the answer to this problem?
d) use the gas law that you indicated in part c above and find the unknown value(be sure to show all of your work)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
You know the right answer?
Questions

Health, 18.09.2021 06:10

Geography, 18.09.2021 06:10

Mathematics, 18.09.2021 06:10


Mathematics, 18.09.2021 06:10

Mathematics, 18.09.2021 06:10

Mathematics, 18.09.2021 06:10

History, 18.09.2021 06:10

Computers and Technology, 18.09.2021 06:10




Chemistry, 18.09.2021 06:10

Mathematics, 18.09.2021 06:10




Mathematics, 18.09.2021 06:20

Mathematics, 18.09.2021 06:20