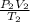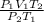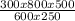Chemistry, 11.10.2019 15:00 raoufhanna
Agas has a volume of 800.0 ml at -23.0 degrees celsius and 300.0 torr. what would the volume of the gas be at 227.0 degrees celsius and 600.0 torr of pressure?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
Agas has a volume of 800.0 ml at -23.0 degrees celsius and 300.0 torr. what would the volume of the...
Questions

Mathematics, 01.08.2019 05:40


History, 01.08.2019 05:40

Biology, 01.08.2019 05:40

Mathematics, 01.08.2019 05:40

Business, 01.08.2019 05:40

Biology, 01.08.2019 05:40


Social Studies, 01.08.2019 05:40

Mathematics, 01.08.2019 05:40

Mathematics, 01.08.2019 05:40

Chemistry, 01.08.2019 05:40

Mathematics, 01.08.2019 05:40

Chemistry, 01.08.2019 05:40

Biology, 01.08.2019 05:40

History, 01.08.2019 05:40




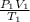 =
=