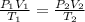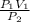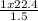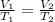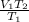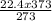Acontainer holds 22.4 l of gas at 1.00 atm and 0.0˚c. a. convert 0.0˚c to kelvin. b. state the combined gas law. c. if the pressure increases to 1.50 atm and the temperature doesn’t change, calculate the new volume. d. if the temperature increases to 100.0 ˚c and the pressure doesn’t change, calculate the new volume.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:40
Which of the following is a testable hypothesis? a. if i brush my teeth, i will get fewer cavities than if i don't brush my teeth. b. green toothpaste tastes better than blue toothpaste or red toothpaste. c. smart, careful, healthy people always brush their teeth. d. it's wrong to not brush your teeth before you have an important conversation with someone.
Answers: 1

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
Acontainer holds 22.4 l of gas at 1.00 atm and 0.0˚c. a. convert 0.0˚c to kelvin. b. state the combi...
Questions



History, 17.03.2020 20:06


Social Studies, 17.03.2020 20:06




Social Studies, 17.03.2020 20:06


Mathematics, 17.03.2020 20:07

Social Studies, 17.03.2020 20:07



History, 17.03.2020 20:07


Chemistry, 17.03.2020 20:07


Biology, 17.03.2020 20:07