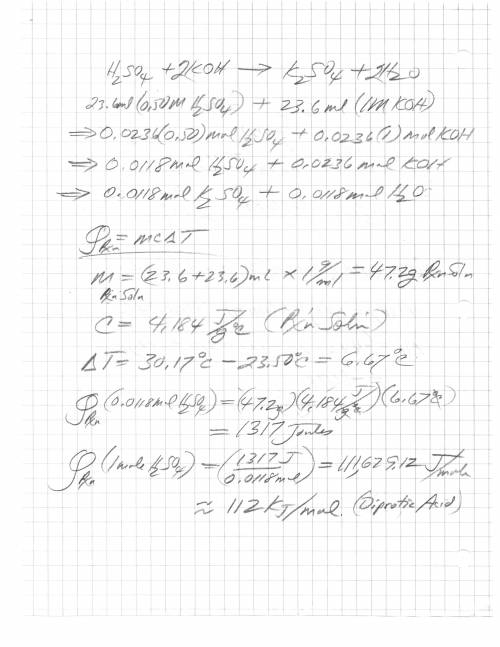Chemistry, 03.02.2020 01:53 wwesuplexcity28
When 23.6 ml of 0.500 m h2so4 is added to 23.6 ml of 1.00 m koh in a coffee-cup calorimeter at 23.50°c, the temperature rises to 30.17°c. calculate δh of this reaction. (assume that the total volume is the sum of the individual volumes and that the density and specific heat capacity of the solution are the same as for pure water.) (d for water = 1.00 g/ml; c for water = 4.184 j/g·°c.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

You know the right answer?
When 23.6 ml of 0.500 m h2so4 is added to 23.6 ml of 1.00 m koh in a coffee-cup calorimeter at 23.50...
Questions


English, 12.10.2020 20:01


Chemistry, 12.10.2020 20:01


English, 12.10.2020 20:01



English, 12.10.2020 20:01


History, 12.10.2020 20:01



Mathematics, 12.10.2020 20:01


Mathematics, 12.10.2020 20:01



Mathematics, 12.10.2020 20:01

Spanish, 12.10.2020 20:01