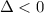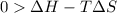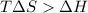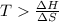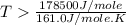For the decomposition of calcium carbonate, consider the following thermodynamic data (due to variations in thermodynamic values for different sources, be sure to use the given values in calculating your answer.): δh∘rxn 178.5kj/mol δs∘rxn 161.0j/(mol⋅k) calculate the temperature in kelvins above which this reaction is spontaneous.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
For the decomposition of calcium carbonate, consider the following thermodynamic data (due to variat...
Questions

History, 19.02.2021 01:00


Mathematics, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00



Social Studies, 19.02.2021 01:00


Social Studies, 19.02.2021 01:00


Biology, 19.02.2021 01:00



Computers and Technology, 19.02.2021 01:00


Mathematics, 19.02.2021 01:00

Chemistry, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00


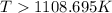
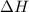 = 178.5 KJ/mole = 178500 J/mole
= 178.5 KJ/mole = 178500 J/mole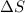 = 161.0 J/mole.K
= 161.0 J/mole.K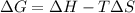
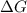 is negative or we can say that the value of
is negative or we can say that the value of