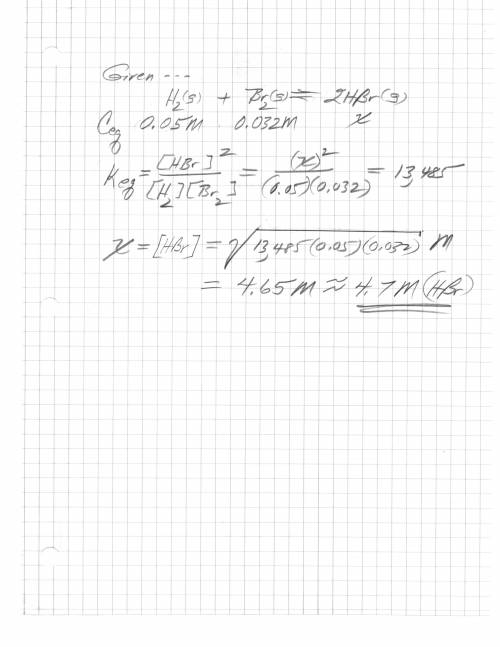
H2 (g) + br2 (g) < => 2 hbr (g)
the equilibrium constant is 13485. at equilibrium...

Chemistry, 23.11.2019 20:31 abronxtale02
H2 (g) + br2 (g) < => 2 hbr (g)
the equilibrium constant is 13485. at equilibrium the h2 concentration is 0.05 m, while the br2 concentration is 0.023 m. calculate the hbr concentration at equilibrium, to 1 decimal. be careful with the units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1
You know the right answer?
Questions



English, 29.10.2020 20:10

Mathematics, 29.10.2020 20:10


Mathematics, 29.10.2020 20:10

English, 29.10.2020 20:10

Mathematics, 29.10.2020 20:10

Mathematics, 29.10.2020 20:10


Mathematics, 29.10.2020 20:10



Mathematics, 29.10.2020 20:10

Social Studies, 29.10.2020 20:10


Mathematics, 29.10.2020 20:10

Mathematics, 29.10.2020 20:10