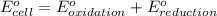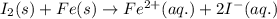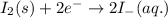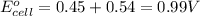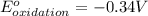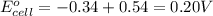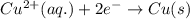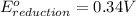Chemistry, 02.07.2019 00:20 cportillo891
Combine the two half-reactions that give the spontaneous cell reaction with the smallest e∘. fe2+(aq)+2e−→fe(s) e∘=−0.45v i2(s)+2e−→2i−(aq) e∘=0.54v cu2+(aq)+2e−→cu(s) e∘=0.34v

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 23.06.2019 10:00
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1
You know the right answer?
Combine the two half-reactions that give the spontaneous cell reaction with the smallest e∘. fe2+(aq...
Questions




Mathematics, 22.07.2019 22:20






Mathematics, 22.07.2019 22:20


Mathematics, 22.07.2019 22:20

Mathematics, 22.07.2019 22:20

Spanish, 22.07.2019 22:20




Mathematics, 22.07.2019 22:20

History, 22.07.2019 22:20

Mathematics, 22.07.2019 22:20

 is
is