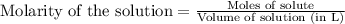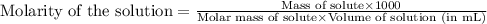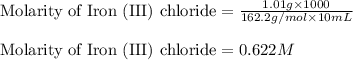Chemistry, 03.07.2019 21:30 lizzyhearts
7. suppose 1.01 g of iron (iii) chloride is placed in a 10.00-ml volumetric flask with a bit of water in it. the flask is shaken to dissolve the solid and the flask is then filled to the mark. what is the molarity of the final solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
7. suppose 1.01 g of iron (iii) chloride is placed in a 10.00-ml volumetric flask with a bit of wate...
Questions


Mathematics, 28.01.2020 15:49





English, 28.01.2020 15:49

Mathematics, 28.01.2020 15:49

Mathematics, 28.01.2020 15:49

Mathematics, 28.01.2020 15:49

Mathematics, 28.01.2020 15:49


Health, 28.01.2020 15:49



Social Studies, 28.01.2020 15:49

Geography, 28.01.2020 15:49

Mathematics, 28.01.2020 15:49