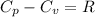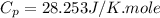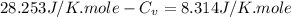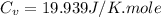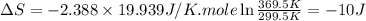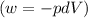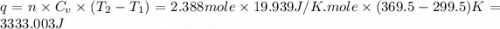Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Ideal gas (n 2.388 moles) is heated at constant volume from t1 299.5 k to final temperature t2 369.5...
Questions

History, 29.04.2021 22:40

Mathematics, 29.04.2021 22:40




Mathematics, 29.04.2021 22:40




Mathematics, 29.04.2021 22:40

Spanish, 29.04.2021 22:40

Mathematics, 29.04.2021 22:40





English, 29.04.2021 22:40


Mathematics, 29.04.2021 22:40

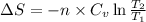
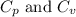 for an ideal gas are :
for an ideal gas are :