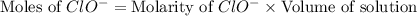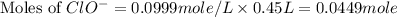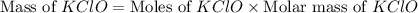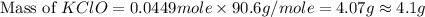Chemistry, 05.07.2019 20:20 TheJanko4526
What mass of potassium hypochlorite (fw-90.6 g/mol) must be added to 4.50 x 10 ml of water to give a solution with ph 10.20? [ka(hcio) 4.0 x 10-8] 0.032g ? 2.4 g 04.1 g 9.1 g 20. g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
You know the right answer?
What mass of potassium hypochlorite (fw-90.6 g/mol) must be added to 4.50 x 10 ml of water to give a...
Questions

History, 02.02.2020 23:43



English, 02.02.2020 23:43

English, 02.02.2020 23:43

Mathematics, 02.02.2020 23:43

Mathematics, 02.02.2020 23:43




Mathematics, 02.02.2020 23:43

Mathematics, 02.02.2020 23:43

Physics, 02.02.2020 23:43

Mathematics, 02.02.2020 23:43






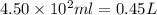
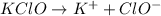
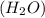 to give,
to give,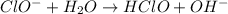
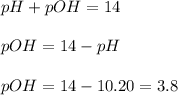
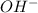 concentration.
concentration.![pOH=-\log [OH^-]](/tpl/images/0055/2981/1fac1.png)
![3.8=-\log [OH^-]](/tpl/images/0055/2981/a714c.png)
![[OH^-]=1.58\times 10^{-4}M](/tpl/images/0055/2981/110da.png)
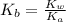
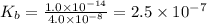
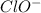 .
.![K_b=\frac{[OH^-][HClO]}{[ClO^-]}](/tpl/images/0055/2981/9b5c8.png)
![[OH^-]=[HClO]=1.58\times 10^{-4}M](/tpl/images/0055/2981/ec220.png)
![2.5\times 10^{-7}=\frac{(1.58\times 10^{-4})^2}{[ClO^-]}](/tpl/images/0055/2981/2ef50.png)
![[ClO^-]=0.0999M](/tpl/images/0055/2981/776df.png)