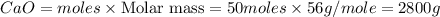Chemistry, 08.07.2019 23:40 treestump090
Limestone (caco3) is decomposed by heating to quicklime (cao) and carbon dioxide. calculate how many grams of quicklime can be produced from 5.0 kg of limestone.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 23.06.2019 15:30
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2
You know the right answer?
Limestone (caco3) is decomposed by heating to quicklime (cao) and carbon dioxide. calculate how many...
Questions

Mathematics, 28.10.2020 20:40


Advanced Placement (AP), 28.10.2020 20:40


Social Studies, 28.10.2020 20:40

History, 28.10.2020 20:40

Physics, 28.10.2020 20:40


Social Studies, 28.10.2020 20:40



Mathematics, 28.10.2020 20:40


Advanced Placement (AP), 28.10.2020 20:40


Social Studies, 28.10.2020 20:40



Social Studies, 28.10.2020 20:40

History, 28.10.2020 20:40

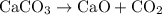
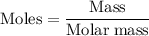
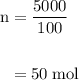
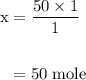

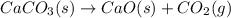
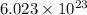 of particles.
of particles.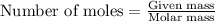
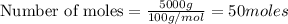
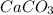 produces = 1 mole of
produces = 1 mole of 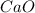
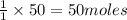 of
of