
Chemistry, 11.07.2019 22:30 IsabellaGracie
For real gases, how does a change in pressure affect the ratio of pvto nrt?
a. the ratio increases as pressure increases.
b. the ratio is constant at all pressures.
c. the ratio decreases as pressure increases.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
For real gases, how does a change in pressure affect the ratio of pvto nrt?
a. the ratio incr...
a. the ratio incr...
Questions


Geography, 13.02.2021 16:40


History, 13.02.2021 16:40

Chemistry, 13.02.2021 16:40

Social Studies, 13.02.2021 16:40

Chemistry, 13.02.2021 16:40

Mathematics, 13.02.2021 16:40



Mathematics, 13.02.2021 16:50


Mathematics, 13.02.2021 16:50

Social Studies, 13.02.2021 16:50

Geography, 13.02.2021 16:50

Biology, 13.02.2021 16:50

Social Studies, 13.02.2021 16:50


Mathematics, 13.02.2021 16:50

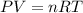
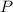 is the pressure of the gas
,
is the pressure of the gas
,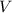 is the volume of gas
,
is the volume of gas
,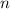 is the number of moles
,
is the number of moles
,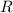 is the gas constant; and
is the gas constant; and 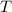 is the temperature of the gas.
is the temperature of the gas.

