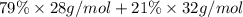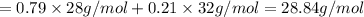Chemistry, 13.07.2019 00:50 ggpro4life3000
Calculate the average molecular weight of air (1) from its approximate molar composition of 79% n2, 21% 02x

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1



You know the right answer?
Calculate the average molecular weight of air (1) from its approximate molar composition of 79% n2,...
Questions


Social Studies, 26.11.2019 06:31


















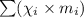
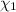 : mole fraction of the 'i' component.
: mole fraction of the 'i' component.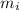 = Molecular weight of i component
= Molecular weight of i component