
Chemistry, 28.08.2019 17:30 themaster66644
If two gases are present in a container, the total pressure in the container is equal to
a. the sum of the pressures that are exerted by each of the two gases.
b. twice the sum of the pressures that are exerted by the individual gases.
c. the sum of the pressures that each gas would exert if they occupied twice the volume.
d. the sum of the pressures that each gas would exert if they occupied half the volume.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 1
You know the right answer?
If two gases are present in a container, the total pressure in the container is equal to
a. th...
a. th...
Questions




Mathematics, 29.09.2019 21:30

Mathematics, 29.09.2019 21:30

English, 29.09.2019 21:30






Mathematics, 29.09.2019 21:30

English, 29.09.2019 21:30


History, 29.09.2019 21:30

English, 29.09.2019 21:30


Social Studies, 29.09.2019 21:30

English, 29.09.2019 21:30

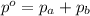
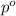 = Total pressure exerted by the gases in the container.
= Total pressure exerted by the gases in the container.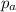 = Partial pressure of gas 'a'
= Partial pressure of gas 'a'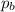 = partial pressure of gas 'b'
= partial pressure of gas 'b'

