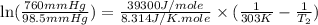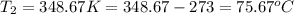Chemistry, 19.07.2019 00:10 melanie7152
The vapor pressure of ethanol is 30°c at 98.5 mmhg and the heat of vaporization is 39.3 kj/mol. determine the normal boiling point of ethanol from this data.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
The vapor pressure of ethanol is 30°c at 98.5 mmhg and the heat of vaporization is 39.3 kj/mol. dete...
Questions

Mathematics, 22.11.2019 20:31

Mathematics, 22.11.2019 20:31

Mathematics, 22.11.2019 20:31




Social Studies, 22.11.2019 20:31

Mathematics, 22.11.2019 20:31





English, 22.11.2019 20:31


English, 22.11.2019 20:31

Business, 22.11.2019 20:31




Mathematics, 22.11.2019 20:31

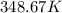 or
or 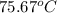
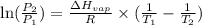
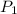 = vapor pressure of ethanol at
= vapor pressure of ethanol at 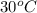 = 98.5 mmHg
= 98.5 mmHg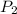 = vapor pressure of ethanol at normal boiling point = 1 atm = 760 mmHg
= vapor pressure of ethanol at normal boiling point = 1 atm = 760 mmHg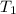 = temperature of ethanol =
= temperature of ethanol = 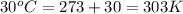
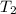 = normal boiling point of ethanol = ?
= normal boiling point of ethanol = ?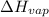 = heat of vaporization = 39.3 kJ/mole = 39300 J/mole
= heat of vaporization = 39.3 kJ/mole = 39300 J/mole