Chemistry, 25.07.2019 03:30 Alphonse8472
Consider the following reaction. how many grams of water are required to form 75.9 g of hno3? assume that there is excess no2 present. the molar masses are as follows: h2o = 18.02 g/mol, hno3 = 63.02 g/mol. no2(g) + h2o(l) → hno3(aq) + no(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1


Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
Consider the following reaction. how many grams of water are required to form 75.9 g of hno3? assum...
Questions



Mathematics, 19.06.2020 03:57

Mathematics, 19.06.2020 03:57


Health, 19.06.2020 03:57

Mathematics, 19.06.2020 03:57









History, 19.06.2020 03:57




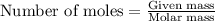 ......(1)
......(1)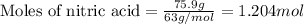
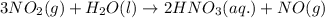
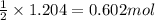 of water.
of water.