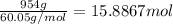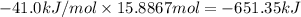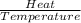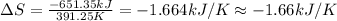Chemistry, 25.07.2019 04:20 thawkins79
The heat of vaporization δhv of acetic acid hch3co2 is 41.0 /kjmol. calculate the change in entropy δs when 954.g of acetic acid condenses at 118.1°c. be sure your answer contains a unit symbol. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

You know the right answer?
The heat of vaporization δhv of acetic acid hch3co2 is 41.0 /kjmol. calculate the change in entropy...
Questions

Biology, 30.09.2019 19:30

Chemistry, 30.09.2019 19:30

English, 30.09.2019 19:30

History, 30.09.2019 19:30




English, 30.09.2019 19:30

Geography, 30.09.2019 19:30

Mathematics, 30.09.2019 19:30


Biology, 30.09.2019 19:30


Law, 30.09.2019 19:30

English, 30.09.2019 19:30

Chemistry, 30.09.2019 19:30

History, 30.09.2019 19:30

Mathematics, 30.09.2019 19:30

English, 30.09.2019 19:30

History, 30.09.2019 19:30